Translationale ALL-Forschung
Our working group conducts preclinical and translational research in acute lymphoblastic leukemia (ALL) biology in different projects:
1) Enhancing antibody immunotherapy by small molecule drugs
Therapy approaches with combinations of chemotherapy and antibodies have improved survival of some patients with B-cell malignancies. However, patients with some diseases like the so called “double-hit-lymphomas” in adulthood or ALL patients harboring a t(17;19) translocation have a very poor prognosis. One cause for the inferior survival is the overexpression of proto-oncogenes likeBCL2 or MCL-1. A potential approach to improve therapy outcome is direct targeting of these proto-oncogenes by small molecule drugs. In our lab, we examine in vitro and in vivo combinations of small molecule drugs and antibodies for patient subgroups, in which therapy options are still limited. The perspective is to translate new targeted therapy strategies by combining small molecule drugs and antibodies into the clinical treatment of patients with B-cell neoplasms.
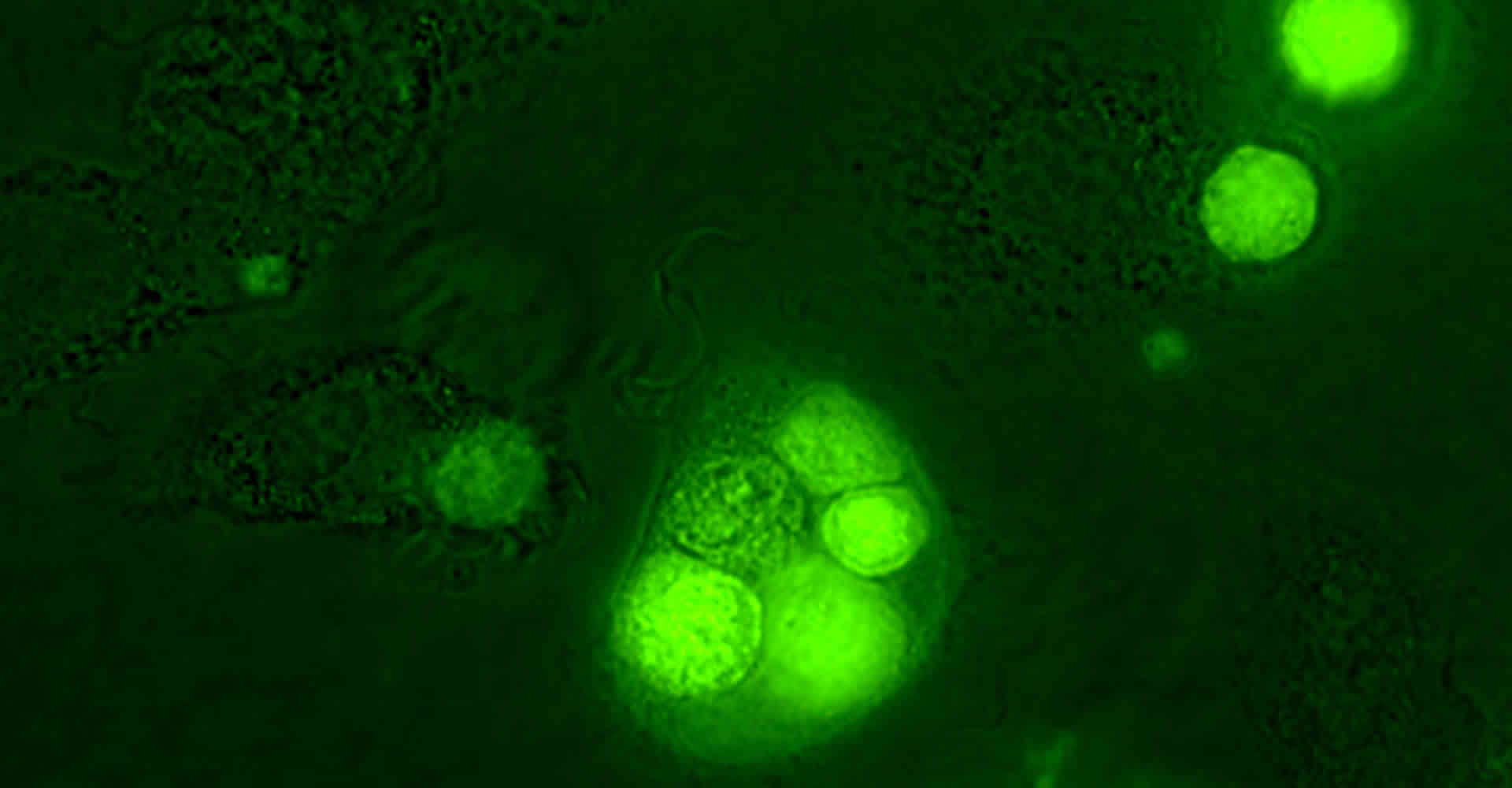
Leukemia cell (green) engulfed by human macrophages after treatment with the Bcl-2 inhibitor venetoclax and the CD20 antibody rituximab
2) Novel immunotherapy approaches in T-ALL
Application of antibodies has evolved as a powerful tool in the treatment of hematological malignancies like acute lymphoblastic leukemia (ALL). Unlike for B-cell precursor (BCP)-ALL, immunotherapeutic interventions in T-cell ALL (T-ALL) are practically non-existent. Moreover, due to increased chemoresistance, chemotherapy strategies at relapse are limited, making novel treatment options urgently needed. Within this project, we examine the potential of antibody-based immunotherapies for T-ALL in order to translate this knowledge into the clinic.
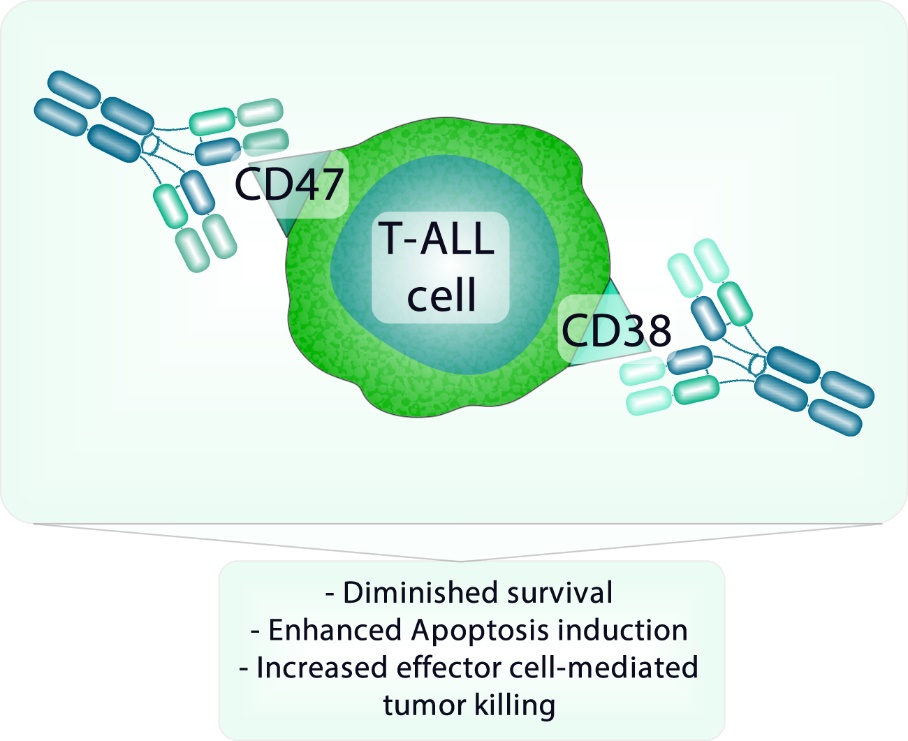
Immunotherapeutic co-targeting of CD47 and CD38 in T-ALL cells
3) Identifying novel mechanisms and targets in CNS leukemia
The infiltration of the central nervous system (CNS) is a major challenge in the therapy of ALL. Due to the high propensity of ALL cells to invade the CNS and low sensitivity of detection methods, ALL patients receive a potent prophylactic chemotherapy and some patients even CNS-radiation. However, if we had specific markers for the detection of ALL cells in the CNS at hand, we could limit intensified therapy only to those patients who would really benefit. To conclusively diagnose and specifically target CNS-ALL, we are working on the mechanisms that are important for CNS involvement in ALL. Our research will identify molecules and pathways that can be used to detect and target ALL cells in the CNS niche.
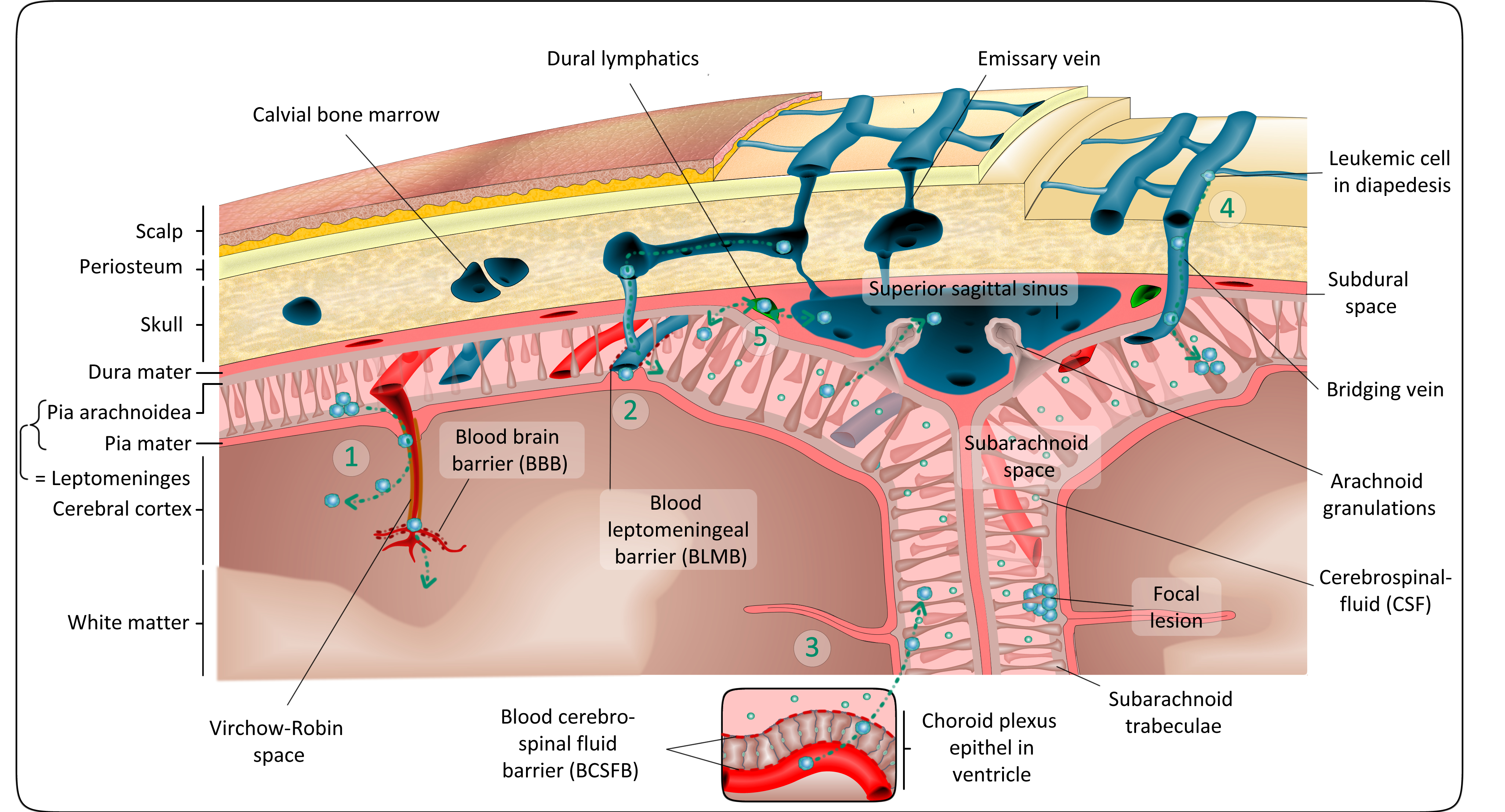
On their way into the CNS, All cells have to overcome different protective barriers (Figure from Lenk et al., Cancer Metastasis Reviews, 2020).
4) CNS targeting antibodies
Currently, CNS-involvement in ALL is mainly treated by aggressive chemotherapy, which is applied systemically or injected directly into the cerebrospinal fluid. A further alternative is radiation therapy. Both kinds of therapy can harm CNS development or even promote secondary tumors, which needs to be prevented, particularly in children. Antibody-based therapies allow efficient killing of ALL cells and tolerable toxicity. Yet, they do not reach the CNS because of its natural protection by the blood brain barrier. In collaboration with the groups of Christian Kellner (Munich) and Matthias Peipp (Kiel), we are attempting to engineer antibodies carrying shuttling-proteins that help antibodies to cross barriers and eradicate ALL cells hiding in protected niches. Generation of more efficient immunotherapy concepts for patients with extramedullary ALL will help solving this important problem.
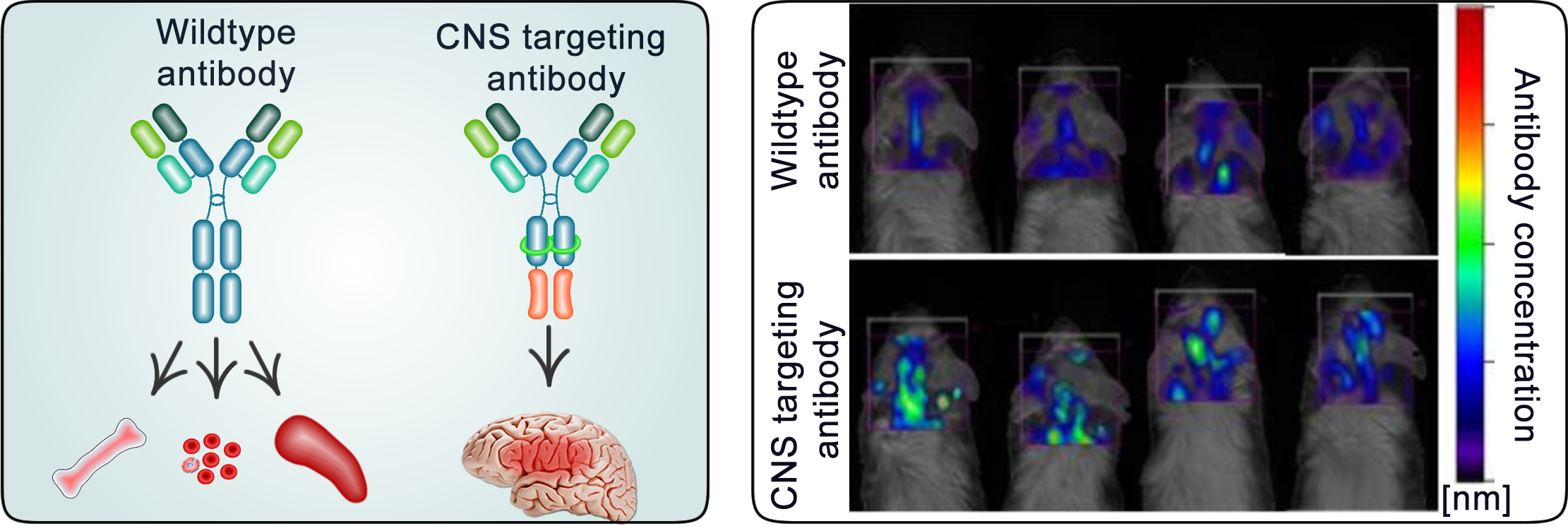
Antibodies are usually not able to penetrate into the CNS. By modifying the structure of an antibody, we can promote its shuttling across the CNS barrier.
5) Preleukemic niches in ALL
ALL cells leaving the bone marrow or the blood circulation in order to infiltrate other organs like the CNS or the testes encounter a hostile environment. In order to survive, the cells have to adapt. When solid tumors, e.g. prostate or breast cancer form metastasis, they send out messenger molecules, causing changes in the composition of the distant organ. These changes occur prior to metastasis formation and are termed “pre-metastatic niche”. In our lab we are investigating corresponding processes in ALL. Understanding the formation of a “pre-leukemic” niche helps us to identify methods and biomarkers for early detection and prevention of organ infiltration in ALL, e. g. to the CNS. Identifying basic biological mechanisms for leukemia invasion into the CNS will help to derive novel therapeutic strategies.
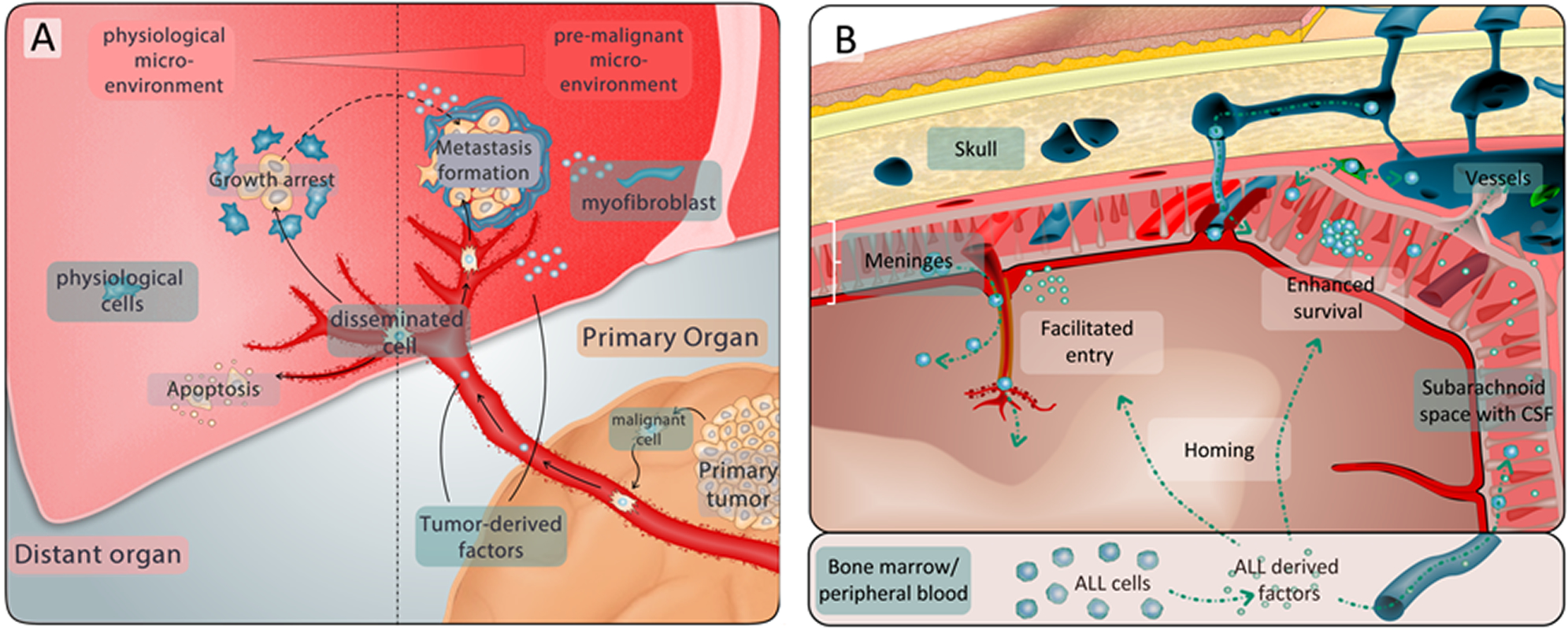
Solid tumors can form a pre-metastatic niche that helps arriving tumor cells to invade and survive in other organs. Similar processes may play a role in ALL and help ALL cells to invade hostile niches.
Publications
https://pubmed.ncbi.nlm.nih.gov/?term=Schewe+D
Funding organizations










