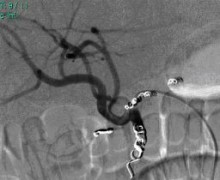
SIRT is based on the introduction of small radioactive beads ("microspheres", product name: SIR-Spheres® with a diameter of c. 35 µm) into the tumour tissue of the liver. After local anaesthesia, the artery in the groin is punctured and a flexible rubber valve - a so-called "catheter sheath" - is inserted into the vessel. A catheter is inserted into the hepatic artery and from there the microspheres are applied. The radioactive element yttrium-90 contained in the spheres radiates for several days (physical half-life approx. 64 hours) over a short distance of a maximum of 11 millimetres with a high local effect ("beta radiation"). This can reduce the risk of damage to surrounding, healthy organ structures.
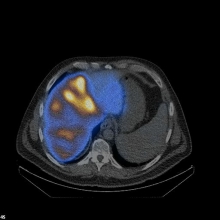
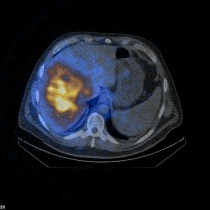
Image: Spect/low-dose CT showing tumor-oriented deposition of the 90Yttrium-labelled SIR-Spheres® in the right liver lobe in a patient with liver metastases from colorectal cancer.