Innovative Imaging Techniques for Neurosurgical Guidance
Micro neurosurgery remains an exceedingly demanding and dexterous fine motor task. Microscope integrated three dimensional imaging techniques which delineate the microstructural composition in depth are missing so far.
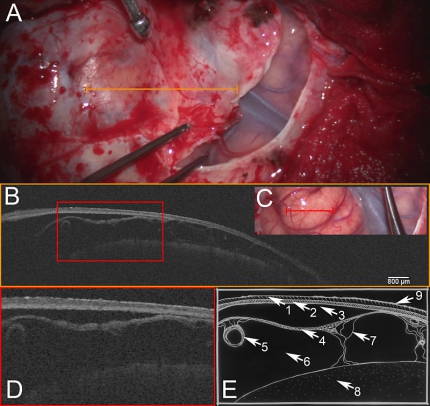
A: Light microscopic image after right fronto-lateral craniotomy, during dissection of dura mater. Opened segment shows sylvian fissure with superficial sylvian veins and temporal as well as frontal brain cortex. Orange line indicates region of scan. B: OCT-scan of dura mater depicting the (1) outer endosteal and (2) inner meningeal layer. Strikingly, a (3) subdural space is present, enabling a clear definition of (2) the inner meningeal dural layer and the (4) arachnoid barrier cell membrane. Furthermore, (5) subarachnoid blood vessels, (6) subarachnoid space, (7) trabecular system, (8) brain cortex and (9) reflection artifacts are depicted by the transdural OCT scan. C: Light microscopic image after dural opening depicting the frontal brain cortex, sylvian fissure and temporal brain cortex. Red line indicates the area of enlarged excerpt. D: Enlarged excerpt demonstrating details of transdural OCT scan. E: Schematic drawing of microstructures: (1) + (2) dura mater, (1) outer endosteal layer, (2) inner meningeal layer, (3) subdural space, (4) subarachnoid space (4) arachnoid barrier cell membrane, (5) subarachnoid blood vessels, (6) subarachnoid space, (7) trabecular system, (8) brain cortex and (9) reflection artifacts.
OCT imaging depends on the detection of back scattered near infrared light and is therefore harmless to biological tissue [1]. Its physical properties allow for microscope integration [2]. This leads to the possibility of contact free three-dimensional, real-time scanning of tissue in the field of view of the surgeon [3]. Penetrating depth depends on optical tissue densities. With approximately 4000 μm in the human cerebral cortex it meets microsurgical requirements [4].
In particular OCT offers an unprecedented axial spatial resolution ranging from 1 - 15 μm –approaching the resolution of conventional histopathology [5]. In vitro recent optical and image processing advancements like automatic serial sectioning of polarization sensitive OCT (asPSOCT) and speckle modulation further increased image quality to display cerebral cortical layers at single cell width [6] [7] [8].
A part from structural imaging adaptations of perfusion-dependent OCT offer the possibility of parallel functional brain mapping [9] [10] [11].Due to the capability of performing “optic biopsies” systems which combine catheter integrated OCT and laser ablation might demonstrate minimal invasive and precise theranostic instruments [12]-[15].
These versatile strengths shed light on future perspectives. Our team validates intraoperative use of microscope integrated OCT for progression of neurosurgical guidance.
[1] E. Beaurepaire, L. Moreaux, F. Amblard, and J. Mertz, “Combined scanning optical coherence and two-photon-excited fluorescence microscopy,” Optics Letters, vol. 24, no. 14, pp. 969–971, 1999.
[2] E. Lankenau, D. Klinger, C. Winter, A. Malik, H. H. Müller, S. Oelckers, H.-W. Pau, T. Just, and G. Hüttmann, “Combining Optical Coherence Tomography (OCT) with an Operating Microscope,” in Advances in Medical Engineering, vol. 114, no. 57, Berlin, Heidelberg: Springer, Berlin, Heidelberg, 2007, pp. 343–348.
[3] E. M. Lankenau, M. Krug, S. Oelckers, N. Schrage, T. Just, and G. Hüttmann, “iOCT with surgical microscopes: a new imaging during microsurgery,” Advanced Optical Technologies, vol. 2, no. 3, pp. 233–239, Jun. 2013.
[4] W. Drexler, “Enhanced Visualization of Macular Pathology With the Use of Ultrahigh-Resolution Optical Coherence Tomography,” Archives of Ophthalmology, vol. 121, no. 5, pp. 695–706, May 2003.
[5] A. K. Ellerbee, “Optical coherence tomography: Technology and applications,” presented at the 2014 IEEE Photonics Conference (IPC), 2014, pp. 7–8.
[6] H. Wang, C. Magnain, R. Wang, J. Dubb, A. Varjabedian, L. S. Tirrell, A. Stevens, J. C. Augustinack, E. Konukoglu, I. Aganj, M. P. Frosch, J. D. Schmahmann, B. Fischl, and D. A. Boas, “as-PSOCT: Volumetric microscopic imaging of human brain architecture and connectivity,” NeuroImage, vol. 165, pp. 56–68, Jan. 2018.
[7] O. Liba, E. D. SoRelle, D. Sen, and A. de la Zerda, “High sensitivity contrast enhanced optical coherence tomography for functional in vivo imaging,” in Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXI, vol. 10053, J. G. Fujimoto, J. A. Izatt, and V. V. Tuchin, Eds. International Society for Optics and Photonics, 2017, p. 100531D.
[8] D. Yecies, O. Liba, A. Zerda, G. G. Neurosurgery, 2018, “Intraoperative Imaging Modalities and the Potential Role of Speckle Modulating Optical Coherence Tomography,” academic.oup.com.
[9] N. Pouratian, S. A. Sheth, N. A. Martin, and A. W. Toga, “Shedding light on brain mapping: advances in human optical imaging,” Trends in Neurosciences, vol. 26, no. 5, pp. 277–282, May 2003.
[10] N. Pouratian, S. Sheth, S. Y. Bookheimer, N. A. Martin, and A. W. Toga, “Applications and limitations of perfusion-dependent functional brain mapping for neurosurgical guidance.,” Neurosurg Focus, vol. 15, no. 1, pp. E2–8, Jul. 2003.
[11] M. Uga, I. Dan, T. Sano, H. Dan, and E. W. M. D, “Optimizing the general linear model for functional near-infrared spectroscopy: an adaptive hemodynamic response function approach,” Neurophoton, vol. 1, no. 1, p. 015004, Aug. 2014.
[12] M. E. Brezinski, G. J. Tearney, B. B. A. O. T. New, 1998, “Optical biopsy with optical coherence tomography,” biophotonics.illinois.edu.
[13] Y. Fan, Y. Xia, X. Zhang, Y. Sun, J. Tang, L. Zhang, and H. Liao, “Optical coherence tomography for precision brain imaging, neurosurgical guidance and minimally invasive theranostics,” BST, vol. 12, no. 1, pp. 12–23, Feb. 2018.
[14] W. Chang, Y. Fan, X. Zhang, and H. Liao, “An Intelligent Theranostics Method Using Optical Coherence Tomography Guided Automatic Laser Ablation for Neurosurgery,” presented at the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 3224–3227.
[15] N. Katta, A. D. Estrada, A. B. McElroy, A. Gruslova, M. Oglesby, A. G. Cabe, M. D. Feldman, R. D. Fleming, A. J. Brenner, and T. E. Milner, “Laser brain cancer surgery in a xenograft model guided by optical coherence tomography,” Theranostics, vol. 9, no. 12, pp. 3555–3564, 2019.
Hartmann, K., Stein, K.-P., Neyazi, B., Sandalcioglu, I.E., 2020a. Microscope integrated optical coherence tomography of a cerebral arachnoid cyst: A new technique to increase intraoperative security. Journal of Clinical Neuroscience 82, 29–31. doi:10.1016/j.jocn.2020.10.008
Hartmann, K., Stein, K.-P., Neyazi, B., Sandalcioglu, I.E., 2020b. Optical coherence tomography of cranial dura mater: Microstructural visualization in vivo. Clin Neurol Neurosurg 200, 106370. doi:10.1016/j.clineuro.2020.106370
Hartmann, K., Stein, K.-P., Neyazi, B., Sandalcioglu, I.E., 2019a. Aneurysm Architecture: First in vivo Imaging of Human Cerebral Aneurysms with Extravascular Optical Coherence Tomography. Cerebrovasc. Dis. 48, 1–6. doi:10.1159/000502450
Hartmann, K., Stein, K.-P., Neyazi, B., Sandalcioglu, I.E., 2019b. First in vivo visualization of the human subarachnoid space and brain cortex via optical coherence tomography:. Therapeutic Advances in Neurological Disorders 12, 175628641984304. doi:10.1177/1756286419843040





